Hazardous isotopes
Our focus here is on the isotopes cesium-137, strontium-90 and iodine-131, since they are relatively volatile and thus can contaminate large areas. In addition, it is these isotopes that accounted for most of the harmful effects following the Chernobyl nuclear accident in 1986.
Cesium-137
Cesium-137 arises as a cleavage product in the nuclear fission of uranium. Cesium-137 reacts further as a β- source to give barium-137. The barium-137 is generated in an excited state (what is referred to as “metastable”), but becomes stable after release of γ-radiation.
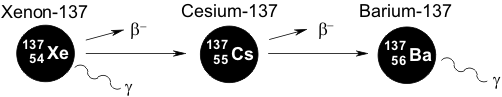
Cesium-137 has a half-life of 30 years; for example, if 1000 cesium-137 atoms are present, then 500 of these will be transformed into barium-137 over a 30-year period. Of the remaining 500 cesium-137 atoms, 250 will be similarly transformed over the succeeding 30-year period.
Barium-137 has a half-life of 2.55 minutes. Since this process takes place so rapidly, from a simplified perspective, the decay of cesium-137 is accompanied by both β-radiation and γ-radiation.
The main problem with cesium-137, which is present in the form of salts, is its high water solubility. Cesium-137 ions are readily distributed in the body, and especially in the muscle tissues. The biological half-life is 110 days. This means that half of the cesium-137 has been excreted again after 110 days.
For the same reasons, however, cesium-137 also finds its way into foodstuffs that have been prepared in contaminated areas.
Iodine-131
Iodine-131 arises as a cleavage product in the nuclear fission of uranium. This β-emitter decays with a half-life of 8 days to metastable xenon, which releases relatively weak γ-radiation.
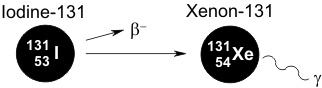
The hazardous β-radiation reaches penetration depths of a few millimeters (~1/8 inch). A problem arises in that thyroid gland hormones contain iodine. Since the body cannot distinguish iodine-131 from stable iodine, it also uses the former in the biosynthesis of these hormones. As the site of this hormone production, the thyroid gland stores these iodine-containing compounds and thus is disproportionately affected by the radiation, which is manifest in the form of thyroid cancer.
Iodine tablets (composed of potassium iodide) are distributed in the vicinity of nuclear power plants so that the iodine reserves in the body will be saturated with stable iodine in the case of a nuclear accident, and thus the subsequent uptake of iodine-131 will be avoided. These tablets should only be taken in the event of an emergency (follow instructions from the local authorities), since the amount of potassium iodide to be used is in the mildly toxic range.
Fresh milk products should be avoided as an additional precaution, since milk contains a significant amount of bound iodine, and the cows will have remained in the pasture as the iodine-131 was spreading through the environment.
Iodine-131 can also be utilized in a form of radiotherapy to kill tumors selectively. It can likewise be employed as an effective treatment for certain thyroid gland disorders. The dose chosen in such a case must be high enough that all the thyroid cells will die. Most of the surrounding tissue is spared due to the shallow penetration depth of the radiation. In principle, it is also possible to use iodine-131 in the preparation of radiopharmaceuticals that can act selectively on tumors. The chemical synthesis of such substances that are labeled with radioisotopes is referred to as “radiolabeling”.
Strontium-90
Strontium-90 is another product that arises from the nuclear fission of uranium. Strontium-90, which is a pure β-emitter, has a half-life of 28 years. This in turn gives rise to yttrium-90, which has a half-life of 64 hours and is a β-emitter in its own right, and the process finally leads to the stable zirconium-90.
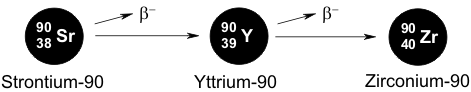
The main problem with strontium-90 comes from its chemical similarity to calcium, so that this isotope is incorporated into bone. As a consequence of the worldwide nuclear weapons testing programs, strontium-90 can readily be detected in the teeth of persons who were born after 1963. A direct relationship has been established between the amounts of incorporated strontium-90 and the likelihood of cancer, and this finally led to the termination of the atomic bomb tests.
